CASE20250423_001
Transjugular Appraoch for Evoque Tanscatheter Tricuspid Valve Replacement in Challenging Anatomy
By Jonathan Fang
Presenter
Jonathan Fang
Authors
Jonathan Fang1
Affiliation
National Heart Centre Singapore, Singapore1,
View Study Report
CASE20250423_001
Tricuspid Valve Intervention - Tricuspid Valve Intervention
Transjugular Appraoch for Evoque Tanscatheter Tricuspid Valve Replacement in Challenging Anatomy
Jonathan Fang1
National Heart Centre Singapore, Singapore1,
Clinical Information
Relevant Clinical History and Physical Exam
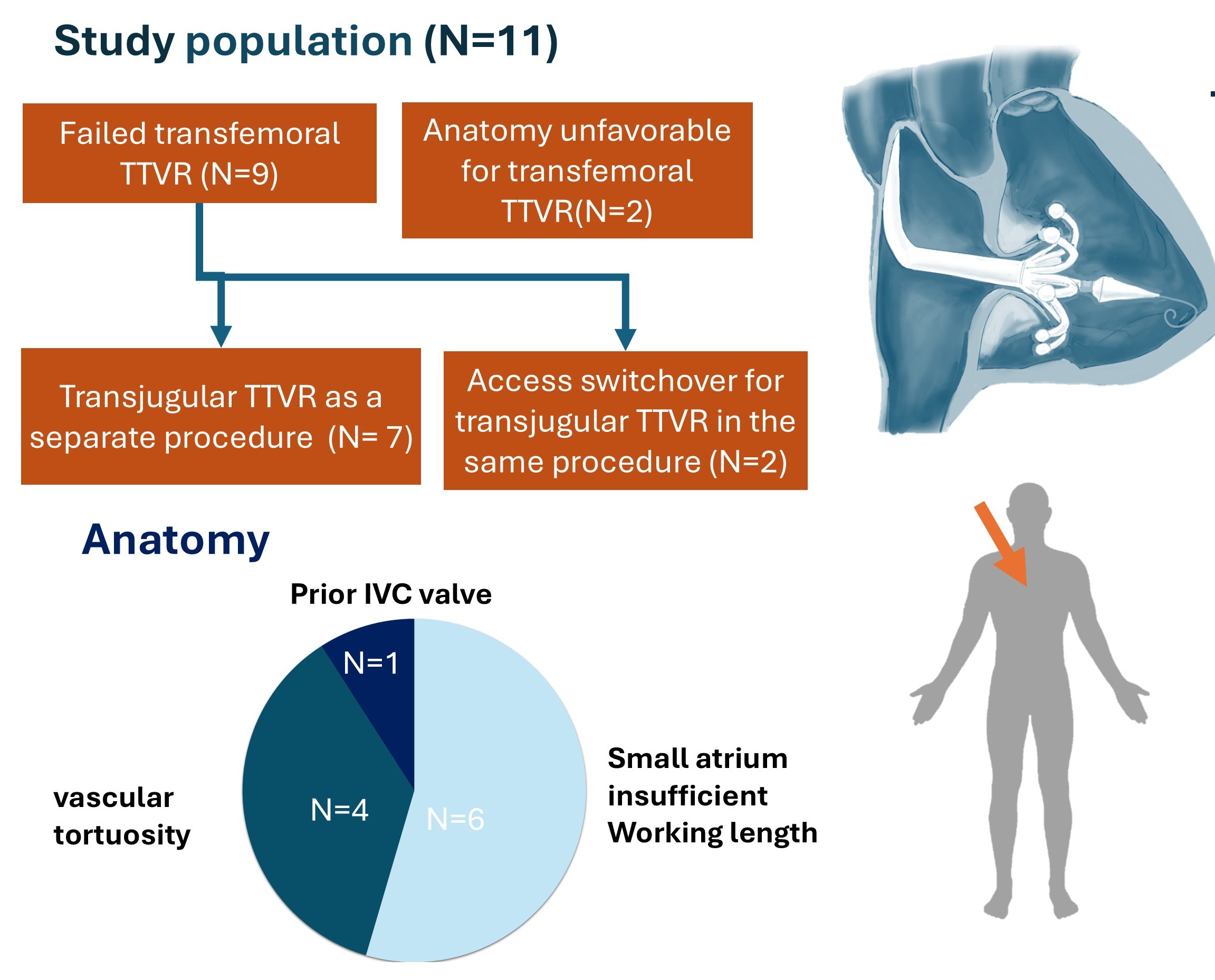
11 patients underwent transcatheter tricuspid valve replacement with the Evoque system via the transjugular access at a tertiary valve center in the United States (Center for Structural Heart Disease, Henry Ford Health, Michigan, USA) after failed transfemoral approach (n=9) or anatomical unsuitability (n=2). These included 6 patients with small atria, 4 patients with venous tortuosity, and 1 patient with a preexisting inferior vena cava valve. We would like to share our technique and results.

Relevant Test Results Prior to Catheterization
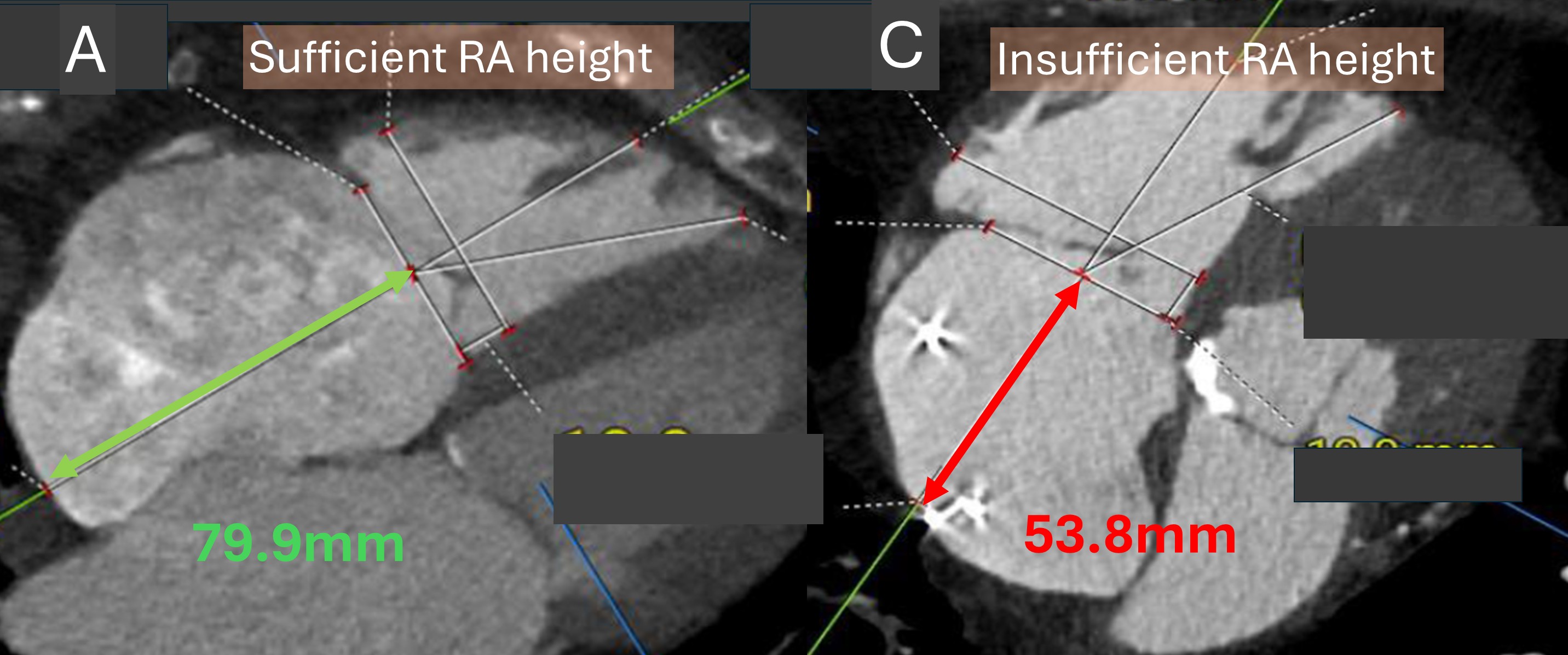
The 6 patients with small atria and right atrial height (RA height) of less than 60mm measured on computed tomography
One patient had a previous caval valve-in-valve placement ( 2 layers ) of balloon-expandable valve inside a stent graft.
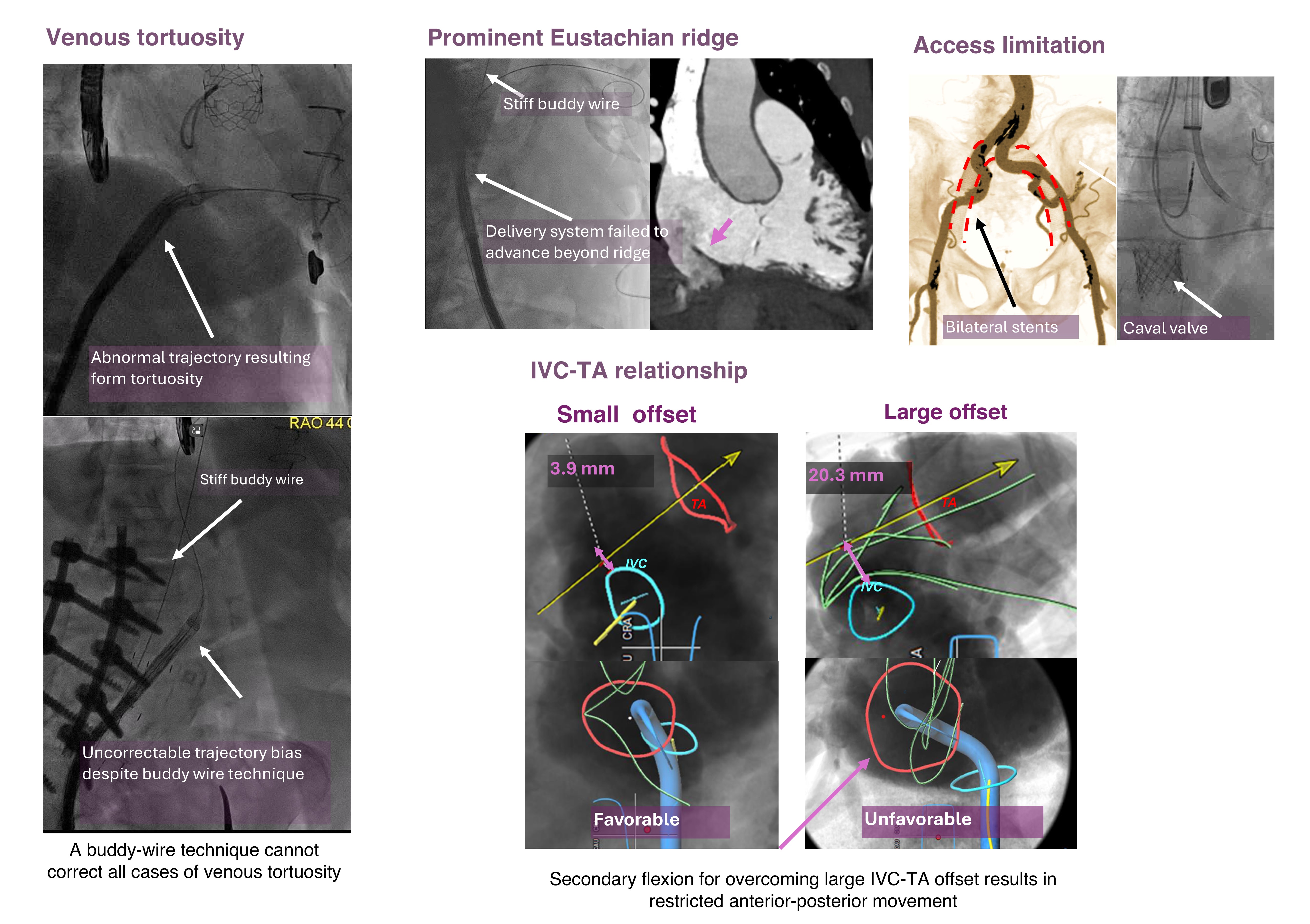
Access-related anatomy leading to unfavorable femoral approach included venous tortuosity, prominent Eustachian ridge, large inferior vena cava-tricuspid annular offset, and access limitation due to vessel diameter.


Relevant Catheterization Findings
Patients with venous tortouosity had demonstrable abnormal bias shown during cahteterization which were not correctable by the use of a stiff buddy wire
Interventional Management
Procedural Step
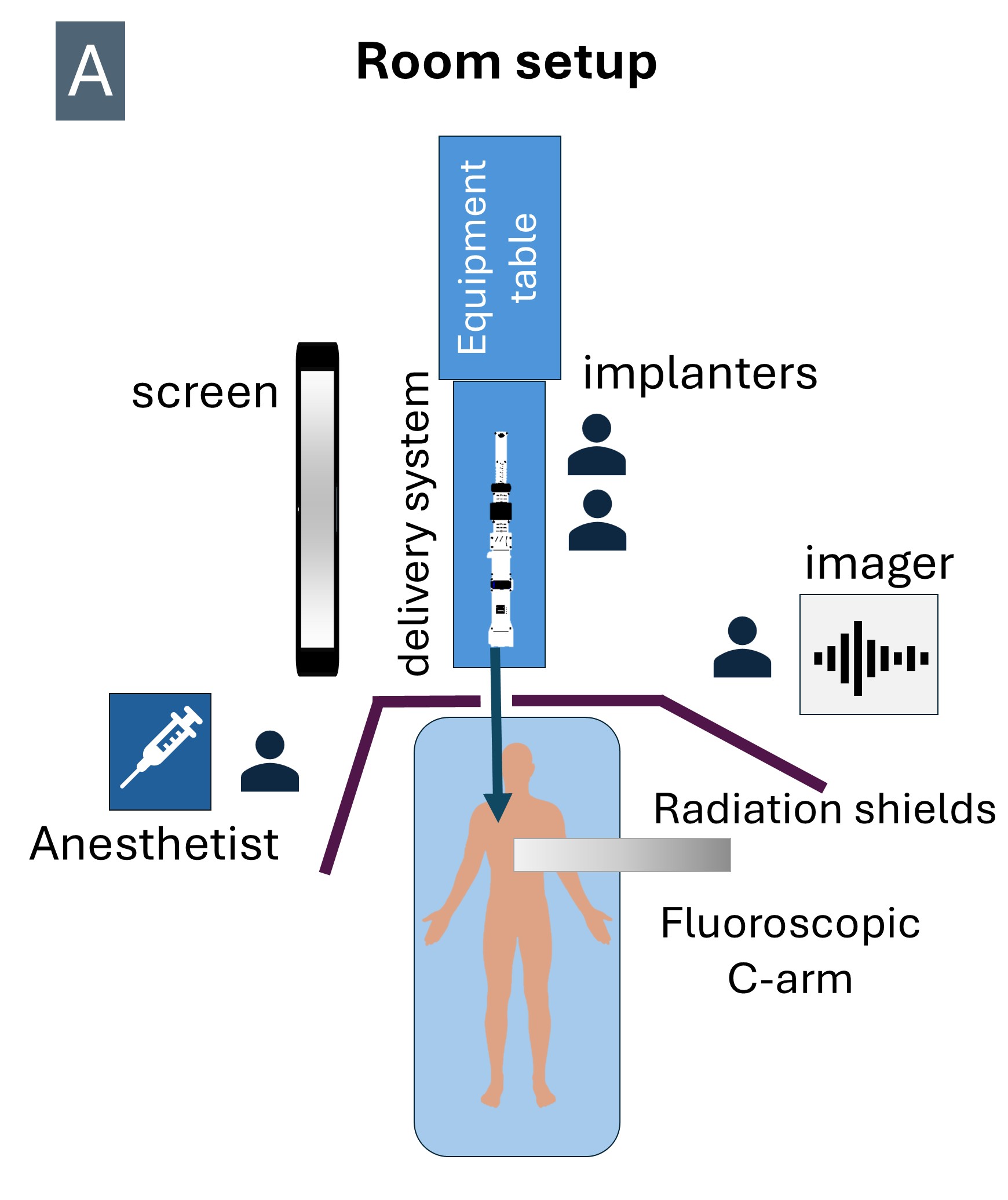
The room is set up with the operators at the head of the patient.Internal jugular vein access was obtained with ultrasound-guided micropuncture and then upsized to 12 French, preclosed with 2 percloses.Under transesophageal echocardiography (TEE) guidance, an 8.5 French steerable introducer (Agilis, Abbott Cardiovascular, USA) was used to place a 0.035-inch Safari extra-small curl wire at the right ventricular apex, ensuring good freedom of movement along the anterior-posterior and septal-lateral planes.The access was then serially dilated, exchanged to a 28 French Evoque delivery system (Edwards Lifesciences, USA), and advanced across the tricuspid annulus. Evoque valve deployment was performed under TEE guidance following the steps of capusle gap formation, anchors exposure, anchors atrial-flipping, ventricular expansion, atrial expansion, and releaseTEE-derived multiplanar-reconstruciton (MPR) was used to guide leaflet capture by nine anchors followed by valve deployementHemostasis was obtained by deploying the percloses +/- figure of 8 suture. Device navigation is noticeably the opposite of that from the transfemoral access.
We performed 11 cases with 100% intraprocedural success, 30-day clinical success, 0% bleeding and vascular complications, and 9.2% pacemaker rate according to TVARC definitions. Median wiring-and-implant time was 66 minutes (IQR 41-103 minutes). TR was reduced to none in 8 patients; mild, 2; moderate, 1 patient.
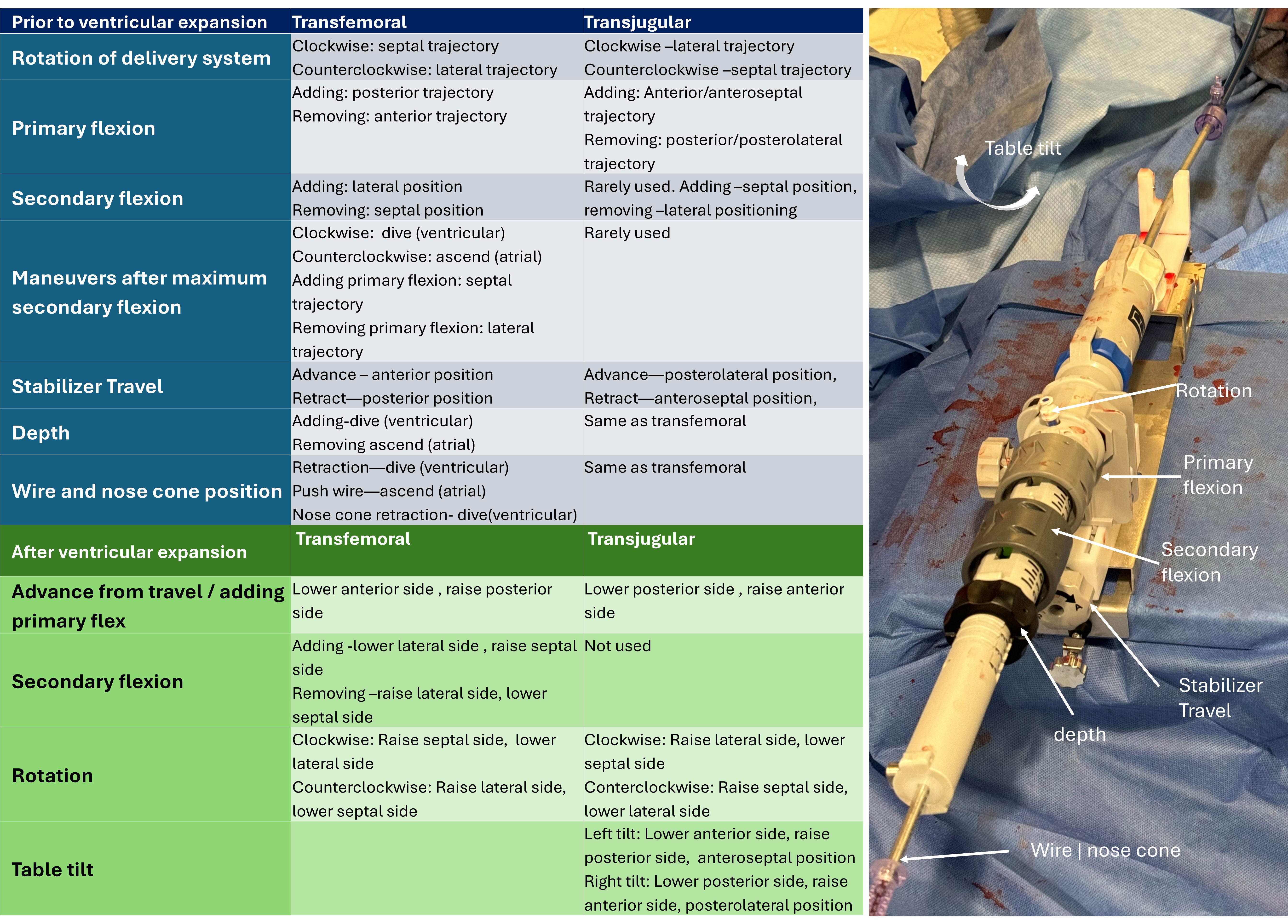
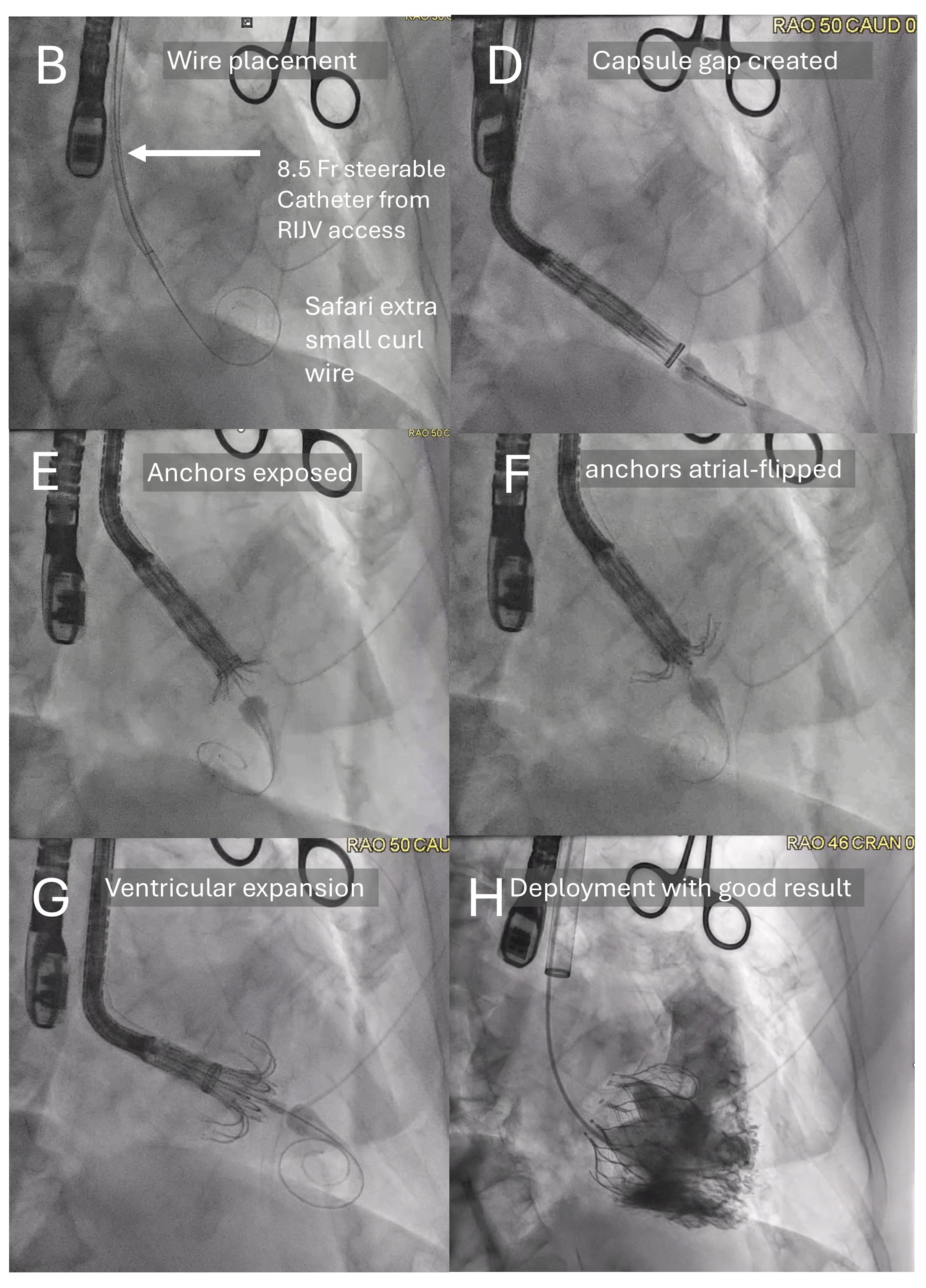
We performed 11 cases with 100% intraprocedural success, 30-day clinical success, 0% bleeding and vascular complications, and 9.2% pacemaker rate according to TVARC definitions. Median wiring-and-implant time was 66 minutes (IQR 41-103 minutes). TR was reduced to none in 8 patients; mild, 2; moderate, 1 patient.



Case Summary
The transjugular approach for tricuspid valve replacement is technically feasible with a high success rate and favorable short-term clinical outcomes for patients with challenging anatomy. This approach may have important implications for patients with a smaller body build, such as East Asian patients, who may have smaller right atria. More studies are required, including a possible randomized controlled trial of a transjugular-first approach for small bodybuild patients when this device becomes available in Asia.
